Our Mission and Activities
Producer of JP Reference Standards and Other Compendial Reference Standards
The Pharmaceutical and Medical Device Regulatory Science Society of Japan (PMRJ) has been registered by the Minister of Health, Labour and Welfare (MHLW) as an organization that produces the Japanese Pharmacopoeia (JP) Reference Standards on the basis of the Ministerial Ordinance (Ordinance of the Ministry of Health, Labour and Welfare No. 117 of 2007), and as such, PMRJ produces and distributes official reference standards used for testing the quality of pharmaceuticals. PMRJ has also been registered as an organization that produces coal-tar color for TLC reference standards in the ministerial ordinance*1 by the MHLW and reference standards for the Japan’s Specifications and Standards for Food Additives*2 by the Prime Minister, and as such, PMRJ produces and distributes those reference standards too. The reference standards produced and distributed by PMRJ are used in tests specified in JP and other official compendia, and by providing the basis for guaranteeing the reliability and objectivity of those tests, those reference standards help ensure and improve the quality of pharmaceuticals and other products in Japan.
*1 Ordinance of the Ministry of Health, Labour and Welfare No. 86 of 2004
*2 Public Notice of the Ministry of Health, Labour and Welfare No. 219 of 2004
The Pharmaceutical Reference Standards Center of PMRJ is responsible for producing and distributing the reference standards based on the direction by the MHLW. PMRJ strives to ensure their quality and provide a stable supply of them by reliably executing the process shown in the diagram below.
How We Work
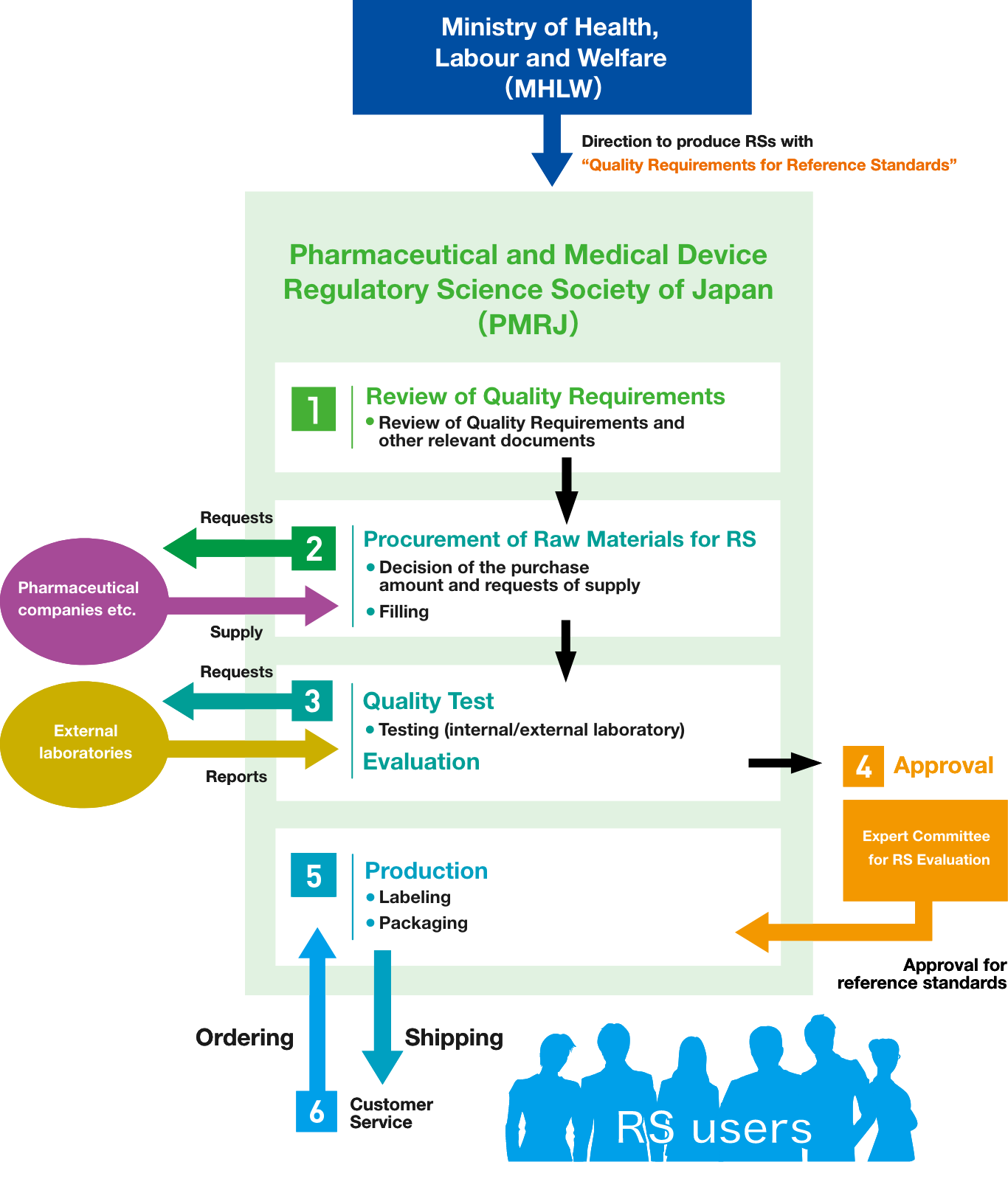
In the establishment of the reference standards, obtaining raw materials of suitable quality for reference standards is critical. The raw materials received by PMRJ are subjected to quality testing by PMRJ and external testing organizations and to be evaluated as reference standard candidate.
As an associate member of the Japanese Pharmacopoeia Expert Committees of the Pharmaceuticals and Medical Devices Agency (PMDA), PMRJ participates in discussions on ensuring the quality and proper use of reference standards and is actively involved in the establishment, production, and distribution of new categories of reference standards. PMRJ also has been striving to understand international trends by interacting with the organizations that manufacture reference standards in Europe and the United States and by participating in international symposia and other such events, and has been working to improve its technical level and strengthen its systems by such measures as obtaining ISO/IEC 17025 laboratory accreditation and participating in proficiency testing. (Quality Assurance)
Distributor of USP Reference Standards, Etc.
Under a contract with the United States Pharmacopeial Convention (USP Convention), PMRJ distributes United States Pharmacopeia (USP) Reference Standards in Japan. In addition, through LGC Standards of Germany, PMRJ distributes pharmaceutical impurity reference materials handled by that company, European Pharmacopoeia (EP) Reference Standards, British Pharmacopoeia (BP) Reference Standards, and World Health Organization (WHO) International Chemical Reference Substances (ICRS).
Technical Training
As one of its educational activities, PMRJ holds training sessions for small numbers of JP users and reference standard users that focus on the JP General Test, such as the Bacterial Endotoxins Test, in order to disseminate practical technical information and exchange technology.